
Contact Info
Adam P. Hitchcock
Canada Research Chair
in Materials Research
CLS-CCRS
B.I.M.R
McMaster University
Hamilton, ON
Canada L8S 4M1
V: +1 905 525-9140
x24729
F: +1 905 521-2773
E: aph@mcmaster.ca
U: unicorn.mcmaster.ca
__________
Research
Group
Opportunities
Publications
Links
_____________

in situ STXM studies of electrochemical reactions
WHO: Adam Hitchcock, Chemistry &Chemical Biology,
BIMR, McMaster University
Zhisheng
Qin, Vinod Prabu (C&CB); Scott Rosendahl (postdoc; now IR
staff scientist, CLS)
Vincent
Lee (postdoc); Hooman Hosseinkhannazer (Norcada Inc)
WHERE: Canadian Light Source CLS-SM
ambient Scanning Transmission X-ray Microscope (a-STXM) 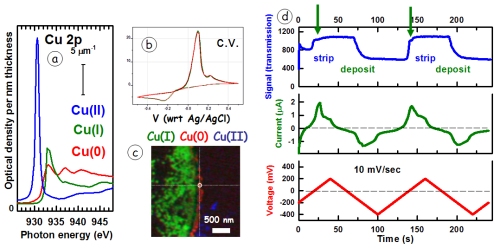
WHEN: 2014-2016 POSTED:
21 Feb 2016
WHAT: Electrochemical reduction of Cu(II) is used to deposit metallic copper in many important technical applications, including integrated circuit interconnects. The reduction of Cu(II) to Cu(0) proceeds via a Cu(I) intermediate, which can be detected under neutral or basic conditions. The quality of the Cu deposit depends strongly on the chemical and electrochemical details of the process. Analytical methods with spatial and temporal resolution that can investigate electrochemical processes are needed to optimize electrochemical processes. Since 2014 we have been working with Norcada Inc and other collaborators to develop improved instrumentation for in situ electrochemical STXM. The figure shows (a) the Cu 2p (L3) spectra, thebasis for differentiating oxidation states of Cu; (b) a typical cyclic voltammogram (ex situ); (c) example of Cu L3 based mapping of Cu(I) intermediate on a custom Norcada in situ holder; (d) in situ time sequence correlating absorption at 934 eV with current and voltage signals in an insitu cv scan. Although the spatial and temporal resolution of competing in situ TEM methods [Schneider et al., Microsc. Microanal. 20, 153-1531 (2014)] are much better, STXM provides detailed chemical speciation through near edge fine structure, and thus is highly complementary.
OTHER INFORMATION: A.P. Hitchcock, Z. Qin, S.M. Rosendahl, V. Lee , M. Reynolds and H. Hosseinkhannazer, In situ electrochemical deposition of Cu studied with scanning transmission x-ray microscopy, Proc. XRM2014. Am. Inst. Phys. Conf Series 1696 (2016) 02003 (1-5) doi: 10.1063/1.4937497
Earlier in situ electrochemical STXM studies were performed on redox chemistry of electrodeposited polyaniline.
© 2016 A.P. Hitchcock / McMaster University
- All Rights Reserved
web site design by Christopher Amis (2002). Last updated on 21 Feb 2016
(aph)

